Draw The Main Lewis Structure Of Nof
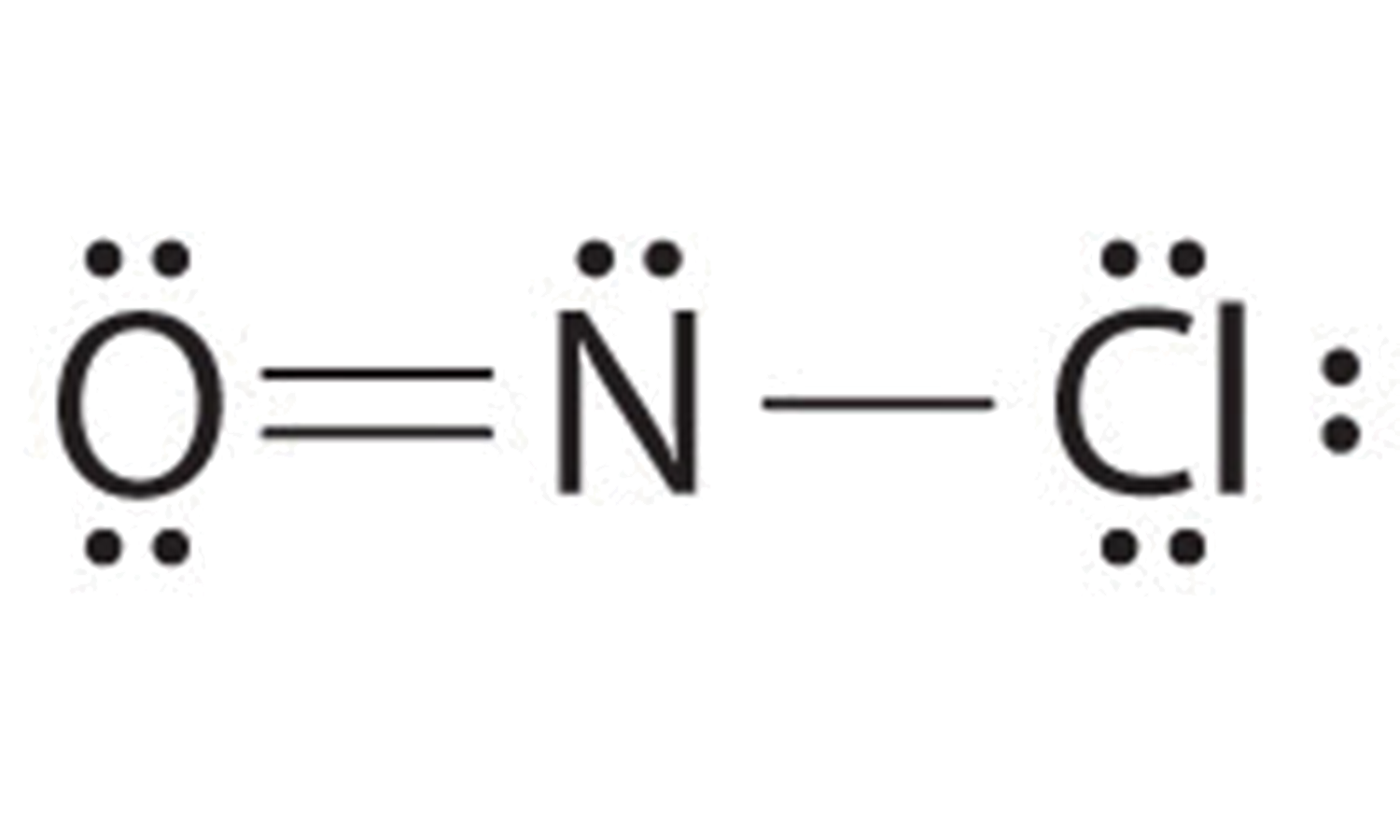
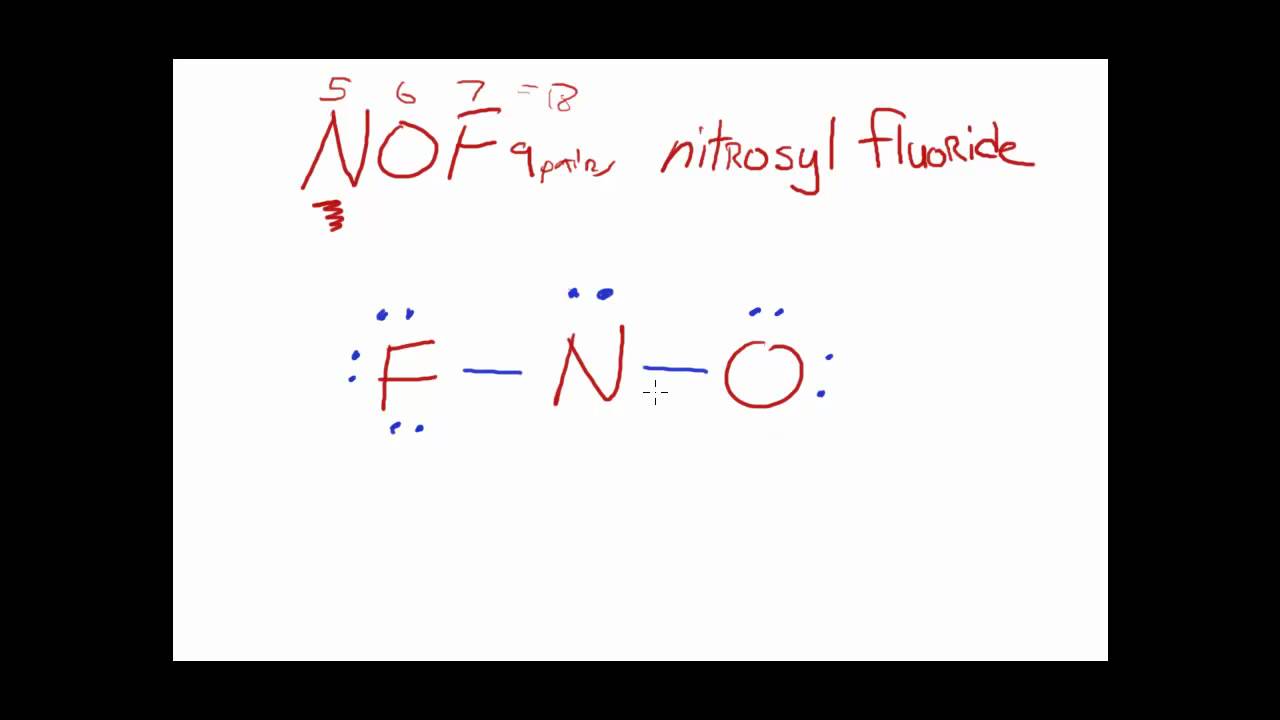
Draw The Main Lewis Structure Of Nof - Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine.the nitrogen is double bonded to the oxygen atom on one sidea. In order to draw the lewis. Can youu imagine learning how to draw lewis structures in less than 60 seconds? Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. Here, the given molecule is nof. Draw the main lewis structure of nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. Web draw the main lewis structure of nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Draw the main lewis structure of nof. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Web the second structure requires more work. In your lab notebook, draw a large picture (lewis structure) of all the molecules (such. Draw the main lewis structure of nof. Web in this video, we are going to look at how to draw lewis structures for nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web the main purpose of lewis structures is to show the arrangement of valence electrons around atoms in a molecule or compound. Draw the lewis structure for nof nitrogen is the central atom draw the molecule by placing atoms on the grid and connecting them with bonds. Web 6 steps to draw the lewis structure of nof step #1: Web steps of drawing nof lewis structure step 1: Web in this video, we are going to look at how to draw lewis structures for nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the.. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. Web drawing the lewis structure for nof. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. Find the total valence electrons in nof molecule. Web steps of drawing nof lewis structure. Lewis structure as we already know is the pictorial representation of electrons around the atoms in a molecule. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Calculate the total number of valence electrons. Draw. Web draw the main lewis structure of nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Let’s apply the concepts we have learned. Here, the given molecule is nof. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Part b draw the main lewis structure of nof. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. In order to draw the lewis. Calculate the total number of valence electrons. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Lewis structure as we already know is the pictorial representation of electrons around the atoms in a molecule. Find the total valence electrons in nof molecule. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Use these steps to correctly draw the nof. Web the second structure requires more work. The nof lewis structure is very similar to nocl and nobr. Part b draw the main lewis structure of nof. Web it is possible to draw a structure with a double bond between a boron atom and a fluorine atom in bf 3, satisfying the octet rule, but experimental evidence indicates the bond.. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The basic idea is to draw. Web drawing the lewis structure for nof. Web nof is a chemical formula for nitrosyl flouride. Web 6 steps to draw the lewis structure of nof step #1: Web a plot of the potential energy of the system as a function of the internuclear distance (figure 5.3.2 ) shows that the system becomes more stable (the energy of the system. Web nof is a chemical formula for nitrosyl flouride. Web the main purpose of lewis structures is to show the arrangement of valence electrons around atoms in a. Draw the main lewis structure of nof. The nof lewis structure is very similar to nocl and nobr. Part b draw the main lewis structure of nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web in this video, we are going to look at how to draw lewis structures for nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Draw the lewis structure for nof nitrogen is the central atom draw the molecule by placing atoms on the grid and connecting them with bonds. Use these steps to correctly draw the nof lewis structure: Part b draw the main lewis structure of nof. Web lewis structure of nof. Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine.the nitrogen is double bonded to the oxygen atom on one sidea. The basic idea is to draw. Calculate the total number of valence electrons. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Determine the number of bonding. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. Web it is possible to draw a structure with a double bond between a boron atom and a fluorine atom in bf 3, satisfying the octet rule, but experimental evidence indicates the bond. Here, the given molecule is nof. Lewis structure as we already know is the pictorial representation of electrons around the atoms in a molecule. Web steps of drawing nof lewis structure step 1: Understand how atoms bond in nof and their unique.Main Lewis Structure Of Nof
Nof Lewis Structure And Resonance Structures
NOF Lewis Structure How to Draw the Lewis Structure for NOF (Nitrosyl
NOF Lewis Structure, Geometry, Hybridization, and Polarity
Structure and Geometry The NOF example YouTube
Draw the main lewis structure of nof. draw nonbonding electrons using
Draw The Main Lewis Structure Of Nof.
NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube
How To Draw The Main Lewis Structure Of Nof learnpedia.click
Lewis Structure
Let’s Apply The Concepts We Have Learned.
Web A Plot Of The Potential Energy Of The System As A Function Of The Internuclear Distance (Figure 5.3.2 ) Shows That The System Becomes More Stable (The Energy Of The System.
Draw Nonbonding Electrons Using The Dot Notation And Bonding Electrons As A Bond.
Find The Total Valence Electrons In Nof Molecule.
Related Post: